
Factor XII deficiency
Individuals with factor XII congenital deficiency (FXII CD) are asymptomatic with respect to a bleeding phenotype. Studies demonstrate that FXII CD is not associated with abnormalities in haemostasis or thrombosis, although levels of FXII between 10%–70% FXII activity have been associated with a higher risk of cardiovascular mortality.1 Because of the lack of obvious symptoms, diagnosis of FXII CD is often missed completely, delayed and/or is discovered incidentally as part of a pre-surgical workup.2-4
During standard laboratory screening, samples from persons with FXII CD show a prolonged activated partial thromboplastin time (aPTT) in one-stage clotting assays, as do deficiencies in FVIII, FIX or FXI. By mixing patient plasma 1:1 with normal plasma, antibodies directed against one of these coagulation factors can be excluded. Individual factor activity assays will reveal low or absent levels of FXII activity in patients with FXII CD.2-5
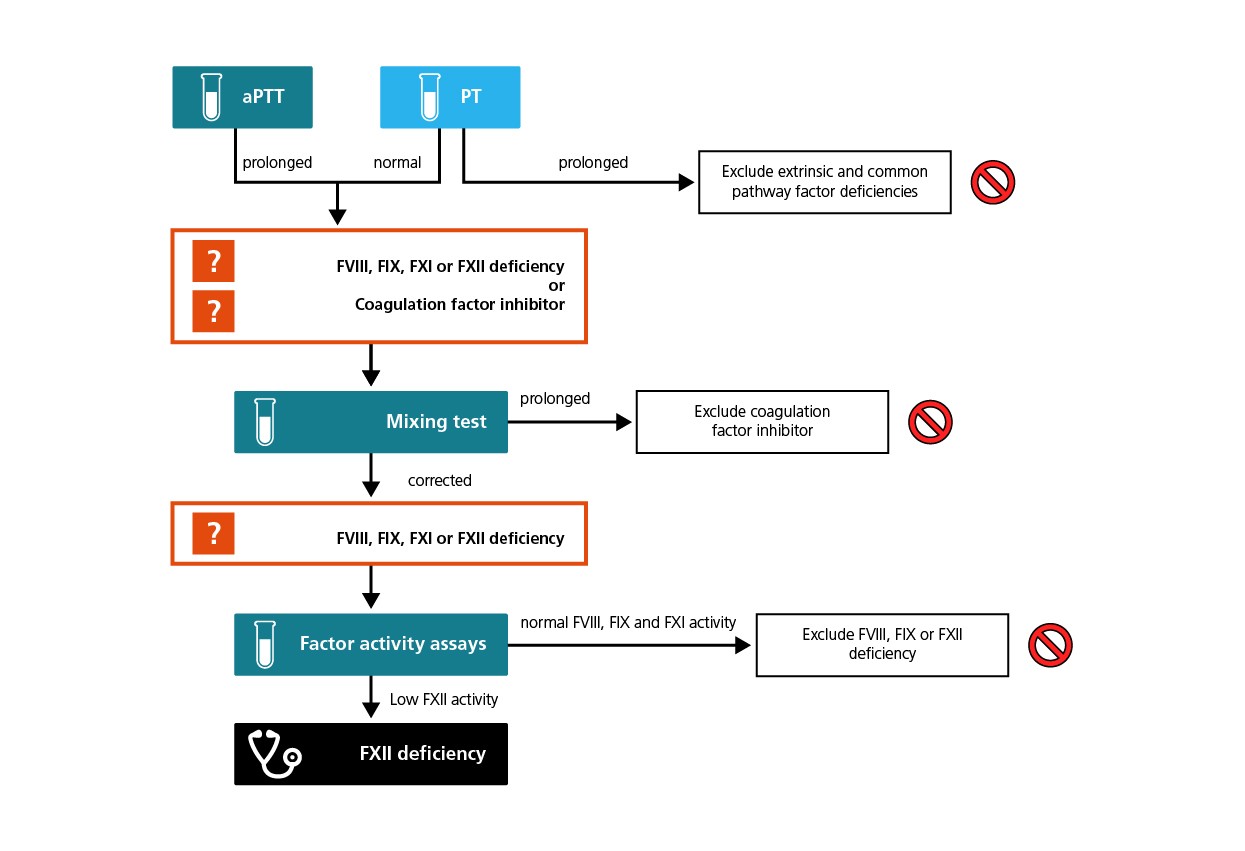
Algorithm for the laboratory diagnosis of FXII deficiency.
Initial laboratory screening
Routine laboratory screening and screening prior to surgery often include activated partial thromboplastin time (aPTT)-based one-stage clotting assays. Patients with FXII CD typically have an isolated abnormal aPTT time and no symptoms or family history of abnormal bleeding. Apart from scientific investigations, a molecular analysis (genotyping) is not indicated for patients with FXII CD.
Mixing tests
Mixing patient plasma samples 1:1 with normal plasma samples can exclude the presence of alloantibodies directed against coagulation factors VIII, IX, XI and XII.
Factor activity assays
Normal activity in individual factor assays can exclude FVIII, FIX or FXI deficiency as the cause of the prolonged aPTT and will show isolated low FXII in patients with FXII CD.
- Endler G, Marsik C, Jilma B, Schickbauer T, Quehenberger P, Mannhalter C. Evidence of a U-shaped association between factor XII activity and overall survival. J Thromb Haemost 2007;5:1143-8.
- Björkqvist J, Nickel KF, Stavrou E, Renné T. In vivo activation and functions of the protease factor XII. Thromb Haemost 2014;112:868-75.
- Renné T, Gailani D. Role of Factor XII in hemostasis and thrombosis: clinical implications. Expert Rev Cardiovasc Ther 2007;5:733-41.
- Muller F, Gailani D, Renne T. Factor XI and XII as antithrombotic targets. Curr Opin Hematol 2011;18:349-55.
- Weidmann H, Heikaus L, Long AT, Naudin C, Schluter H, Renne T. The plasma contact system, a protease cascade at the nexus of inflammation, coagulation and immunity. Biochim Biophys Acta Mol Cell Res 2017;1864:2118-27.
